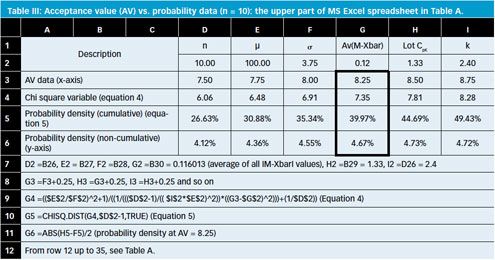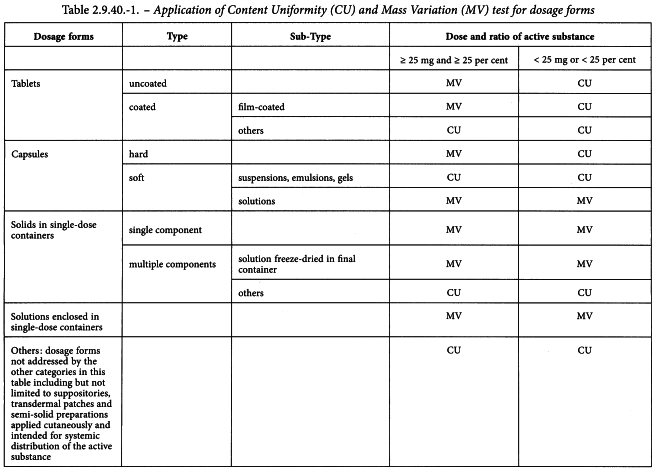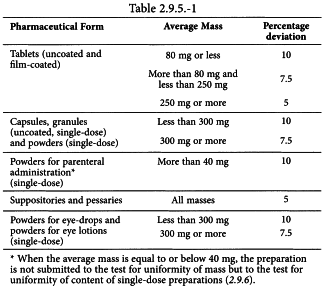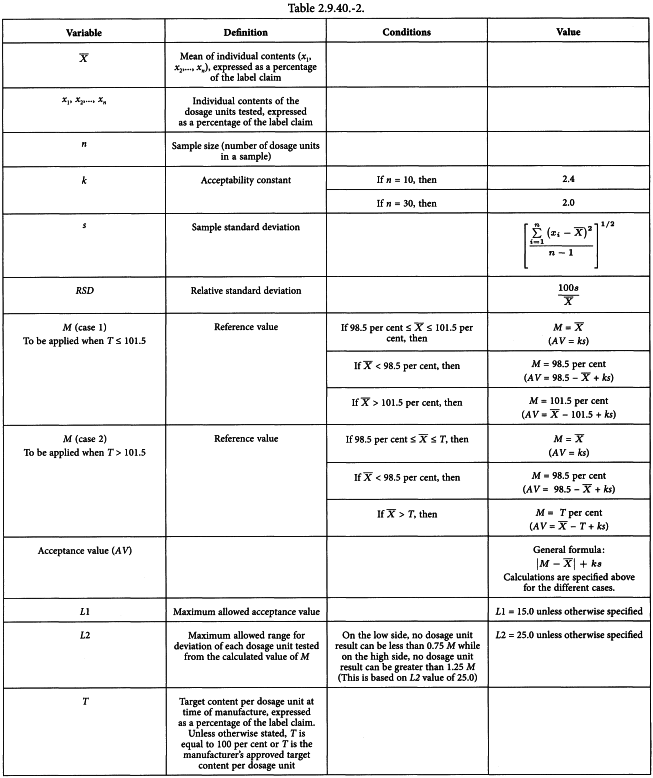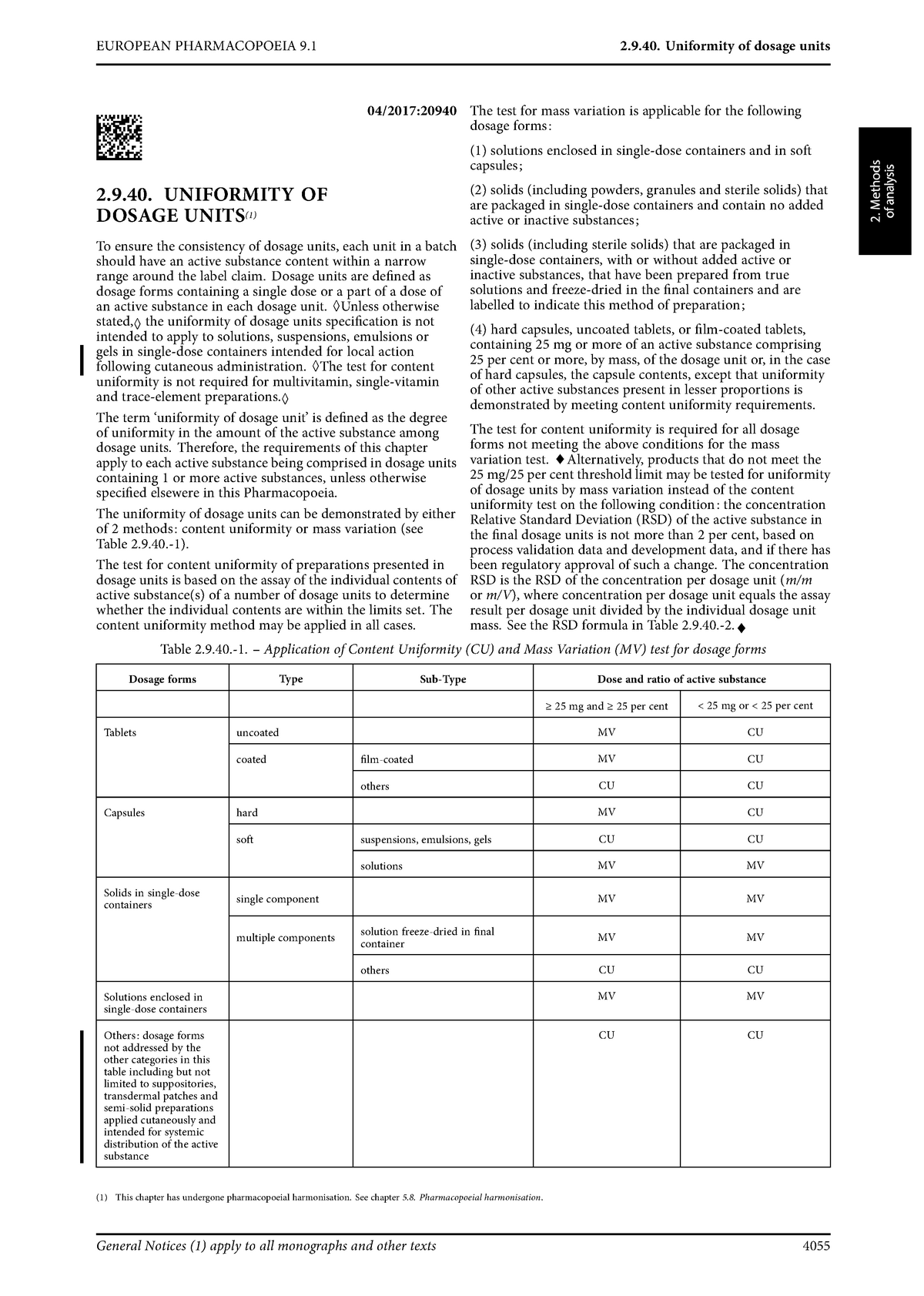
Uniformity of dosage units - EUROPEAN PHARMACOPOEIA 9 2.9. Uniformity of dosage units 04/2017: 2.9. - Studocu

Statistical Properties of Large Sample Tests for Dose Content Uniformity - Øyvind Holte, Matej Horvat, 2014
ICH guideline Q4B annex 6 to note for evaluation and recommendation of pharmacopoeial texts for use in the ICH regions on unifor

PDF) Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method

ASTM E2810-11 - Standard Practice for Demonstrating Capability to Comply with the Test for Uniformity of Dosage Units

Quantification of atropine sulphate monohydrate and obidoxime dichloride in two‐chamber autoinjectors for accessing uniformity of dosage - Spreitzer - Analytical Science Advances - Wiley Online Library